Rely on the Libre 3 Plus sensor for the most accurate and consistent readings over 15 days.1
The FreeStyle Libre 3 Plus sensor conveniently works with the current FreeStyle Libre 3 app.◊◊ Your patients will see real-time glucose readings to help them make more informed decisions3 in the moment.
Accurate, stable, and consistent for 15 days1
The world’s smallest 15-day sensor1,2
The Libre 3 Plus is smaller than the size of a £1 coin.1
Unmatched 15-day wear performance1
Demonstrates a high degree of accuracy consistently for up to 15 days.1
Real-time glucose
readings every minute
Your patients will see real-time readings sent directly to their smartphones◊◊ to help them make more informed decisions3 in the moment.
Partnerships with insulin pumps
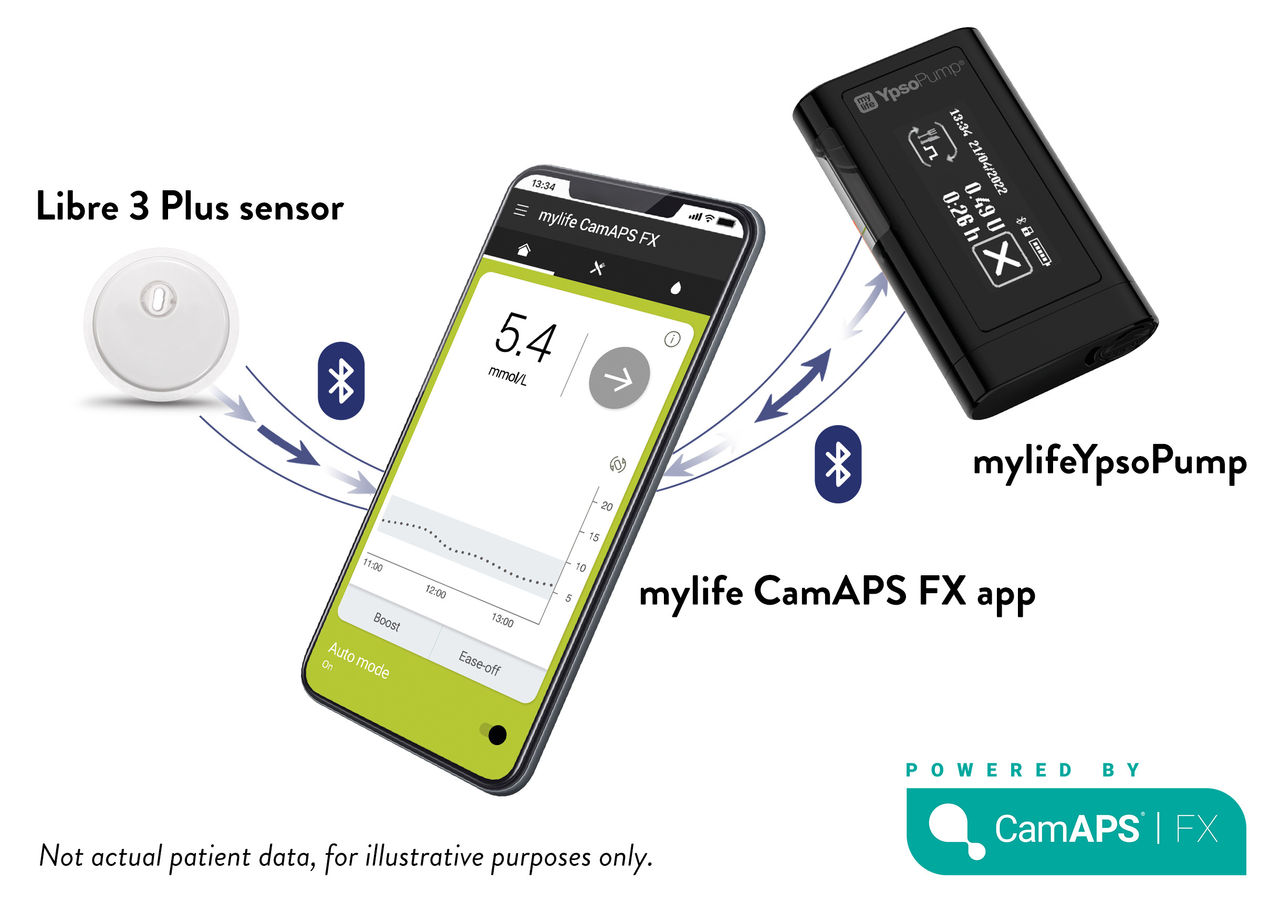
The Libre 3 Plus sensor is authorised to work with the mylife Loop automated insulin delivery system, including mylife CAM APS® FX app and mylife YpsoPump insulin pump. For use of the Libre 3 Plus sensors with the mylife Loop, refer to the labeling provided with the mylife CamAPS FX app.
Indicated for ages 2+^
The Libre 3 Plus sensor is indicated for patients 2 years and older whereas the Libre 3 sensors are indicated for patients 4 years and older.^
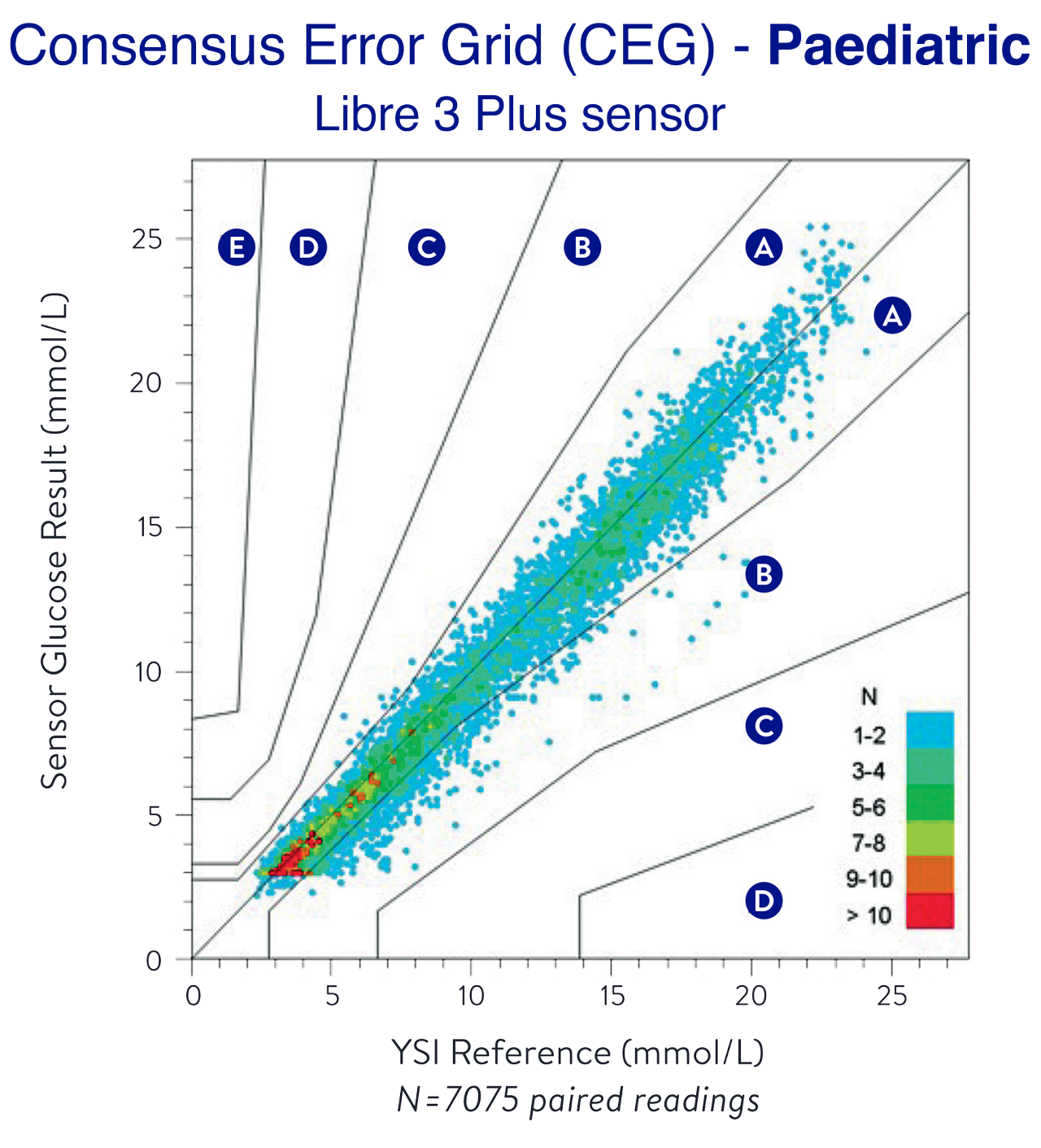
Proven 15-day accuracy1

MARD = MARD=Mean Absolute Relative Difference; YSI=Yellow Springs Instrument
93% in Zone A alone, posing no effect on clinical action1
Strong low-end accuracy (<3.9 mmol/L)1
MARD = MARD=Mean Absolute Relative Difference; YSI=Yellow Springs Instrument
94.3% in zone A, posing no effect on clinical action1
Strong low-end accuracy (<3.9 mmol/L)1
Libre 3 Plus sensor works with
mylife Loop Automated Insulin Delivery System
Authorised to work with the Libre 3 Plus sensor
Minute-to-minute glucose readings— combined with the mylife Loop automated insulin delivery system—for easier4 diabetes management for your patients.
Compatible products
Libre 3 App
Real-time glucose readings from the Libre 3 and Libre 3 Plus sensor are automatically updated every minute and sent directly to the Libre 3 app◊◊ on your patients' smartphones.
LibreView
Using LibreView,₼ our secure5 cloud-based system, patients can upload their data to share with their doctor and care team. You will be able to access more in-depth reports to support you in making treatment decisions for your patients.
LibreLinkUp
Family members can see the glucose levels of their child or elderly parent right on their smartphone if connected with the LibreLinkUp app.§
References & Disclaimers
Images are for illustrative purposes only. Not real patient or data.
1. Data on file. Abbott Diabetes Care, Inc
2. Among patient-applied sensor.
3. Fokkert, M. et al. Improved Well-being and Decreased Disease Burden After 1-Year Use of Flash Glucose Monitoring (FLARE NL-4)." BMJ Open Diabetes Research and Care 7, no 1 (2019): e000809. https://doi.org/10.1136/bmjdrc-2019-000809.
4. Based on product features including up to 15-day wear period, automatic readings every minute, accuracy data, and single-app setup with AID systems.
5. LibreView is ISO27001/27018/27701 certified and HITRUST CSF Certified.
◊◊ The FreeStyle Libre 3 app is only compatible with certain mobile devices and operating systems. Please check our website for more information about device compatibility before using the app. Sharing of glucose data requires registration with LibreView.
* No YSI measurements were obtained for ages 2-5 years.
^ For children aged 2-12, a caregiver at least 18 years old is responsible for supervising, managing, and assisting them in using the FreeStyle Libre 3 system and interpreting its readings.
₼ The LibreView data management software is intended for use by both patients and healthcare professionals to assist people with diabetes and their healthcare professionals in the review, analysis and evaluation of historical glucose device data to support effective diabetes management. The LibreView software is not intended to provide treatment decisions or to be used as a substitute for professional healthcare advice.
§ The FreeStyle LibreLinkUp app is only compatible with certain mobile device and operating systems. Please check www.LibreLinkUp.com for more information about device compatibility before using the app. Sharing of glucose data requires registration with LibreView. The LibreLinkUp mobile app is not intended to be a primary glucose monitor: home users must consult their primary device(s) and consult a healthcare professional before making any medical interpretation and therapy adjustments from the information provided by the app.
The FreeStyle Libre 3 and Libre 3 Plus sensors are authorised to work with the mylife Loop automated insulin delivery system, including the mylife CAM APS® FX app and mylife YpsoPump insulin pump. For use of the FreeStyle Libre 3 sensors with the mylife Loop, refer to the labeling provided with the mylife CAM APS® FX app. Mylife and YpsoPump are registered trademarks of Ypsomed AG. CamAPS is a registered trademark of CamDiab Ltd. Other trademarks are the property of their respective owners.
ADC-106651 v2.0
.svg)




