To evaluate the efficacy of the FreeStyle Libre system in GDM management.
FLAMINGO study
FreeStyle Libre use in gestational diabetes mellitus
Evaluating the FreeStyle Libre system in pregnant women with gestational diabetes
Hyperglycaemia affects 15% of pregnant women in Europe1 and is a risk factor for maternal and foetal complications during pregnancy, including foetal macrosomia.2
FLAMINGO was a randomised controlled trial (RCT) that analysed the effects of the FreeStyle Libre flash glucose monitoring system compared to self-monitoring of blood glucose (SMBG) on glycaemic control and perinatal outcomes in pregnant women with gestational diabetes mellitus (GDM).3
FreeStyle Libre use impacted glycaemic control, dietary habits and reduced the incidence of foetal macrosomia compared with SMBG in pregnant women with GDM.
Study objective
Study design & study population
FLAMINGO was a non-blinded RCT designed to assess the efficacy of flash glucose monitoring in GDM.
Study enrolment occurred between March, 2020 and October, 2022 at the 1st Department of Obstetrics and Gynaecology, Medical University of Warsaw, Poland.
One hundred pregnant women (aged >18) diagnosed with GDM between 24–28 weeks of gestation were enrolled and randomly assigned (1:1) to either the intervention group (flash glucose monitoring, n=50, out of which 49 completed the study) or the control group (SMBG, n=50).
The intervention group used the FreeStyle Libre system for the first 4 weeks after GDM diagnosis, followed by SMBG for the remainder of the pregnancy, whilst the control group used SMBG throughout the entire study period until birth.
After recruitment, the participants were followed up at four visits (2 and 4 weeks after recruitment, 34–36 weeks of gestation and postpartum). During the three follow up visits, clinical and laboratory data were assessed, including glucose concentrations, qualification for insulin therapy and dosage, diet habits, physical activity and gestational weight gain. HbA1c levels and ultrasound estimated foetal weight were measured at the second and third follow up visits.
Perinatal outcomes, including weeks of gestation, route of birth, newborn weight and neonatal hypoglycaemic events, were assessed in the 4th follow up visit.
Key Inclusion criteria*
- Participant’s age >18 years old
- Diagnosed with GDM between 24–28 weeks of gestation (fasting plasma glucose 5.1– 6.9 mmol/L; 1-hour glucose concentration ≥10.0 mmol/L; or 2-hour glucose concentration 8.5 –11.0 mmol/L)
- Singleton pregnancy
Key Exclusion criteria*
- Foetal malformations
- Pre-gestational diabetes
- Physiologic risk factors and medications with potential impact on pregnancy outcome
- Multiple pregnancy
 Primary outcome
Primary outcome
- Mean fasting and 1-hour postprandial glycaemia† during the first 28 days following GDM diagnosis.
 Key secondary outcomes
Key secondary outcomes
Maternal outcome
- HbA1c concentrations (2, 4 and 8 weeks after recruitment)
- Number of patients requiring insulin therapy and dosage of insulin (2, 4 and 8 weeks after recruitment).
- Hypoglycaemic episodes (<3.9 mmol/L) during the first 4 weeks of the study.
- Physical activity (number of footsteps per day) in the first 4 weeks of the study.
- Diet modifications assessed at weeks 2, 4 and 8 after recruitment.
- Gestational weight gain assessed at weeks 2, 4 and 8 after recruitment.
- Mode of delivery.
Neonatal outcome
- Foetal birth weight 24–72 hours after delivery.
- Incidence of foetal macrosomia.
- Neonatal hypoglycaemia 24–72 hours after delivery.
Study results
Key patient baseline characteristics
The study included 100 participants randomised into two study arms (FreeStyle Libre, intervention arm [n=49]); SMBG, control arm [n=50]). There was no significant difference in maternal baseline characteristics between the two groups.
![The study included 100 participants randomised in to two study arms (FreeStyle Libre, intervention arm [n=49]); SMBG, control arm [n=50]). There was no significant difference in maternal baseline characteristics between the two groups.](/content/dam/adc/pro/countries/uk-en/impact-study/Table-of-the-baseline-demographic-and-clinical-characteristics.png)
![The study included 100 participants randomised in to two study arms (FreeStyle Libre, intervention arm [n=49]); SMBG, control arm [n=50]). There was no significant difference in maternal baseline characteristics between the two groups.](/content/dam/adc/pro/countries/uk-en/impact-study/Table-of-the-baseline-demographic-and-clinical-characteristics.png)
IQR: interquartile range.
 Primary outcome
Primary outcome
Glycaemic control
- There was no significant difference in mean (SD) fasting glucose concentration between the two groups during the first 4 weeks following GDM diagnosis (p=0.437), wherease the mean postprandial glycaemia, with lower concentration in the control group (p = 0.011). Delta mean glucose concentrations were significantly reduced in the study group in comparison to the control group, with lower delta fasting and delta postprandial mean glycaemia in the flash glucose monitoring group in the third and fourth week followinb the inclusion of the study (Fig 1).
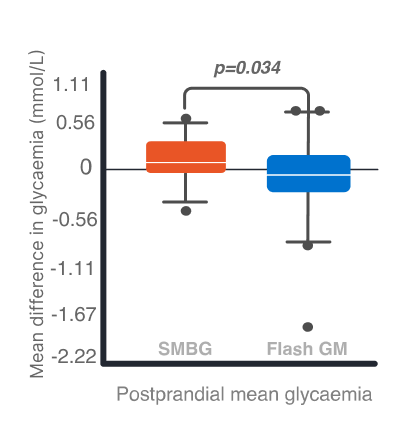

Fig. 1. Mean difference in glycaemia between 14–28 and 1–13 days following GDM diagnosis. Left: Fasting glycaemia. Right: Postprandial glycaemia. A p-value of <0.05 was a cut-off for significant difference. Error bars, standard deviation.
 Secondary outcome measures
Secondary outcome measures
Eating Assessment Test (EAT) score from patient-reported questionnaire did not differ at the recruitment visit, but was significantly higher in the intervention group between 34 and 36 weeks of gestation (37 points [34–39] vs. 34 points [33–37], p = 0.017).
No significant differences between groups were seen in:
Maternal outcome
- Delta HbA1c concentration (defined as difference between HbA1c measured between 34–36 weeks of gestation and A1c measured at the recruitment visit): control group 0.1% (−0.1–0.2) vs intervention group 0.05% (−0.2–0.2); p=0.546.
- Insulin therapy (32% from the control group vs. 30.61% from the intervention group; odds ratio 1.09, 95% CI 0.47–2.57).
- Long-acting insulin dosage at follow-up visit between 34–36 weeks of gestation (p=0.199).
- Incidence of caesarean section (odds ratio 0.84, 95% CI 0.38–1.87).
- Weeks of gestation at birth (p=0.872).
- Gestational weight gain between recruitment and 3rd follow up visit (p=0.682).
- Weeks of gestation at birth (p=0.872).
- Physical activity at 3rd follow up visit (p=0.302).
Nonatal outcome
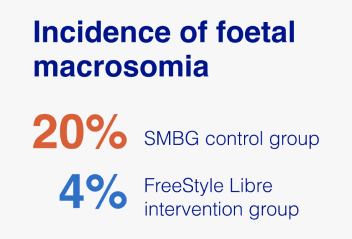
- Incidence of foetal macrosomia (>4000 g) was significantly higher in the SMBG control group compared to the FreeStyle Libre intervention group (20% vs. 4.08%, OR 5.62, 95% CI 1.16−27.22).
- Large for gestational age neonates (defined as birthweight > 90 percentile) and neonatal hypoglycaemia also appeared more often in the control group, but the differences were not statistically significant (OR 2.38, 95% CI 0.69–8.22, and OR 1.29, 95% CI 0.50–3.28, respectively). Median birthweight percentiles did not differ significantly between the groups (p = 0.697).
Study limitations
- The study assessed glycaemic control for the first 4 weeks after GDM diagnosis and did not include glycaemic fluctuations that might occur later in pregnancy.
Summary
The FLAMINGO study was a non-blinded RCT designed to assess the efficacy of flash glucose monitoring for the management of GDM. It was the first long-term assessment of glycaemia with the FreeStyle Libre system in GDM.
The FreeStyle Libre system resulted in a higher EAT score, indicating better diet modifications after GDM diagnosis.
The FreeStyle Libre system significantly reduced the incidence of foetal macrosomia.
Further studies are required to evaluate the effect of the FreeStyle Libre system on improving perinatal outcomes in pregnancies complicated by GDM.
References & Disclaimers
Images are for illustrative purposes only. The data from this study was collected using the FreeStyle Libre system. The FreeStyle Libre 2 and FreeStyle Libre 3 systems have the same features as the FreeStyle Libre system with real-time glucose alerts. Therefore, the study data are applicable to both products.
* A full list of inclusion/exclusion criteria can be found at Majewska et al., 2023.3
† Glycemia results analysis according to Polish Society of Obstetricians and Gynecologists recommendations for gestational diabetes mellitus.
1. International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. https://www.diabetesatlas.org. Accessed 27 Feb 2025.
2. Ye, Wenrui et al. (2022): Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. In BMJ 377, pp. ie067946. DOI: 10.1136/bmj-2021-067946.
3. Majewska, Agata et al. (2023): Flash glucose monitoring in gestational diabetes mellitus (FLAMINGO): a randomised controlled trial. In Acta Diabetologica 60 (9), pp. 1171–1177. DOI: 10.1007/s00592-023-02091-2.
ADC-97349 v2.0
.svg)

