References & Disclaimers
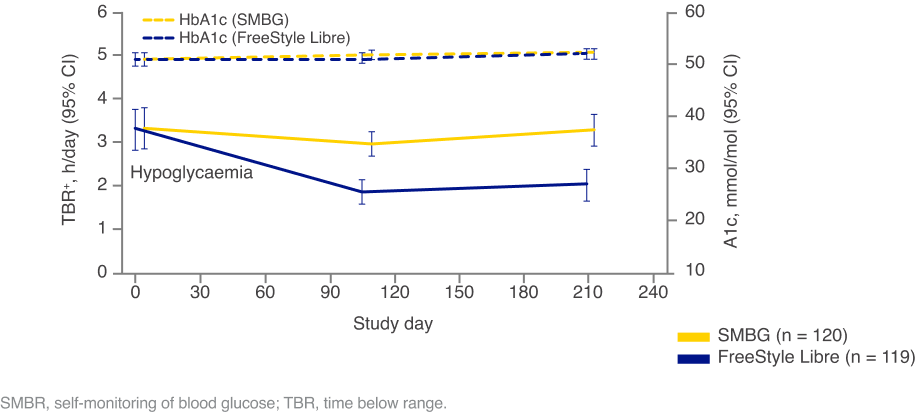
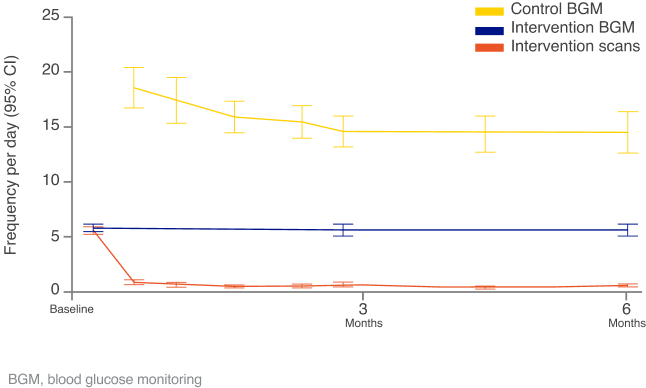
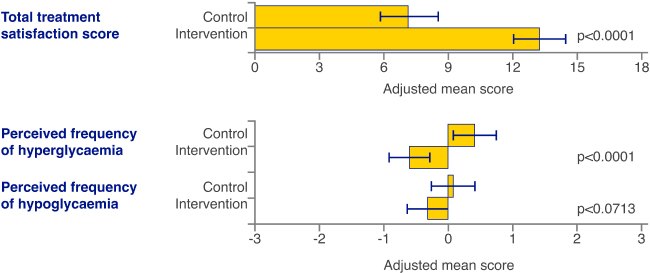
Images are for illustrative purposes only. Not real patient data. Study data collected with the FreeStyle Libre system and is applicable to the FreeStyle Libre 2 & FreeStyle Libre 3 systems based on technological similarities.
* A full list of inclusion criteria, exclusion criteria, and primary and secondary outcomes can be found at https://clinicaltrials.gov/study/NCT02232698.
† Episode defined as at least two consecutive readings at 15 min intervals, outside the predefined glucose range. The end of an episode was one reading at or higher than the threshold.
1 Bolinder J, et al. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254-2263. doi: 10.1016/S0140-6736(16)31535-5.
.svg)