A search for relevant publications was conducted across key abstracting and indexing databases.* The search covered the period from 2013 until December 31st 2020.
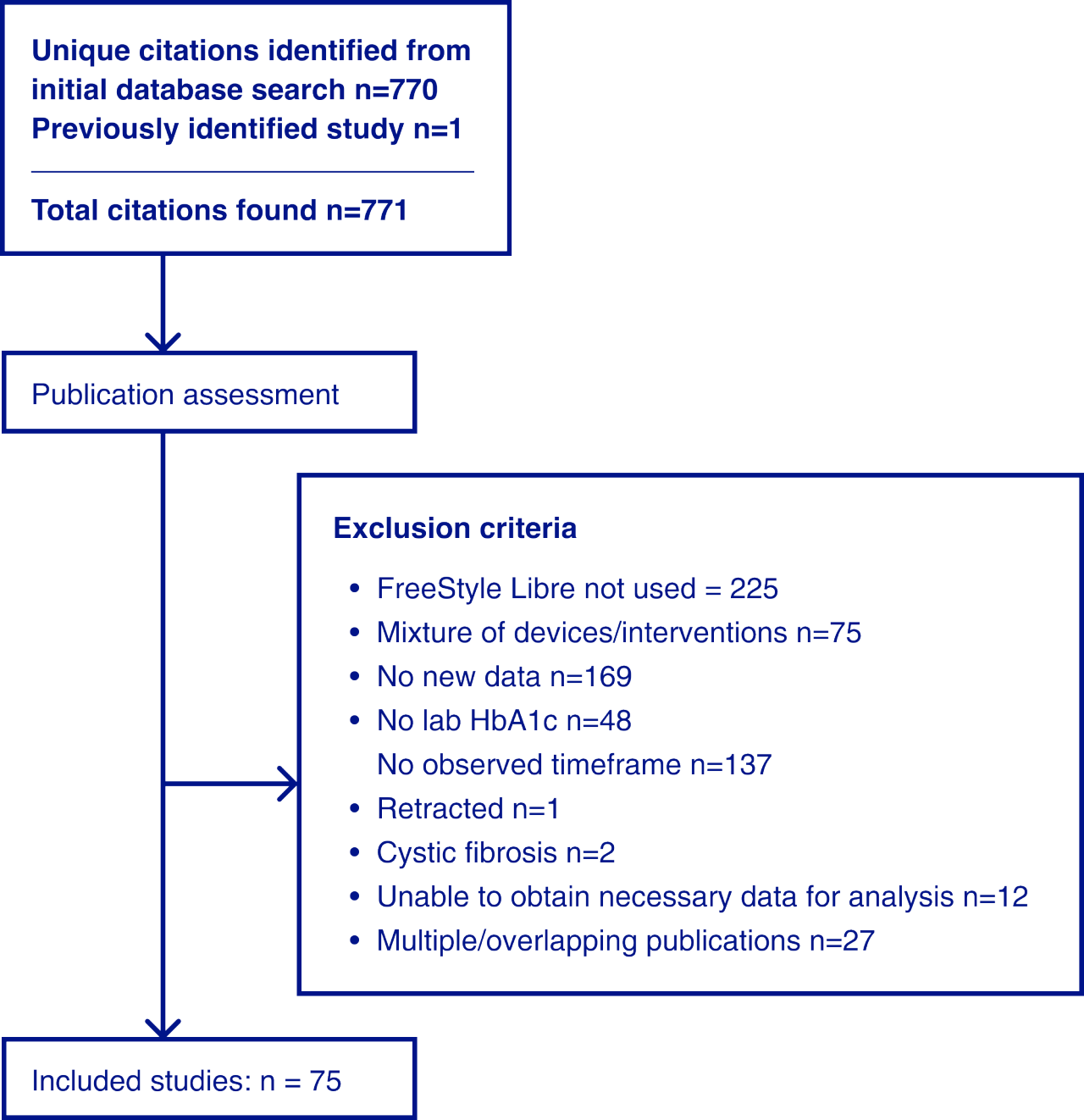
75 studies were identified that reported longitudinal laboratory HbA1c data in participants with T1D or T2D using the FreeStyle Libre system over periods from 1 to 24 months. All studies reported data in a manner that could be used in a statistical meta-analysis.
For randomised controlled trials within the analysis set, within-intervention arm results were included, but not the between-arm results reported previously. All studies were reported as absolute change from baseline to ensure a consistent approach and to reflect the real-world patient- centred outcome.
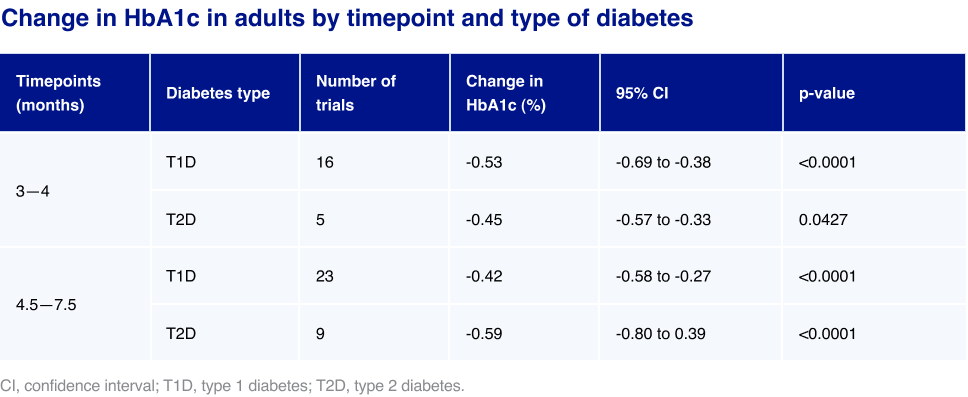
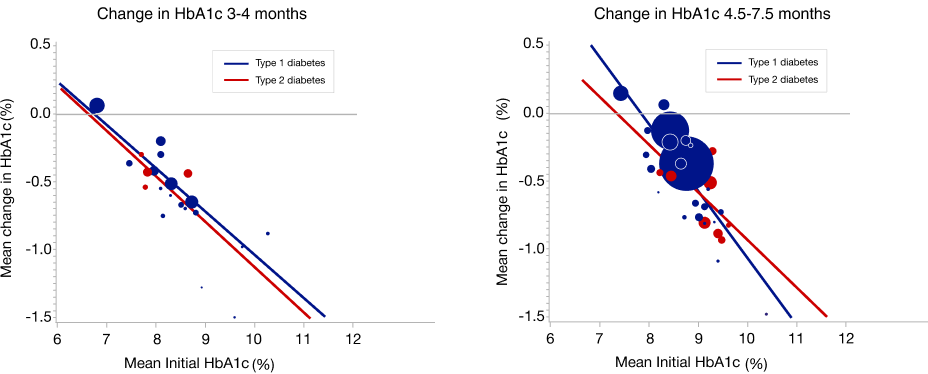
The meta-analysis centred on observed change in HbA1c at 3–4 months and 4.5–7.5 months, as well as a longitudinal analysis up to 24 months. Mean change in HbA1c adjusted for initial HbA1c was estimated using random effects meta- regression versus initial HbA1c by type of diabetes with type of diabetes, month, and interaction of type of diabetes and month as fixed effects. Separate analyses were conducted for adults and children.
Study results were published online in the expert journal Diabetes Therapy in April, 2022.1
.svg)