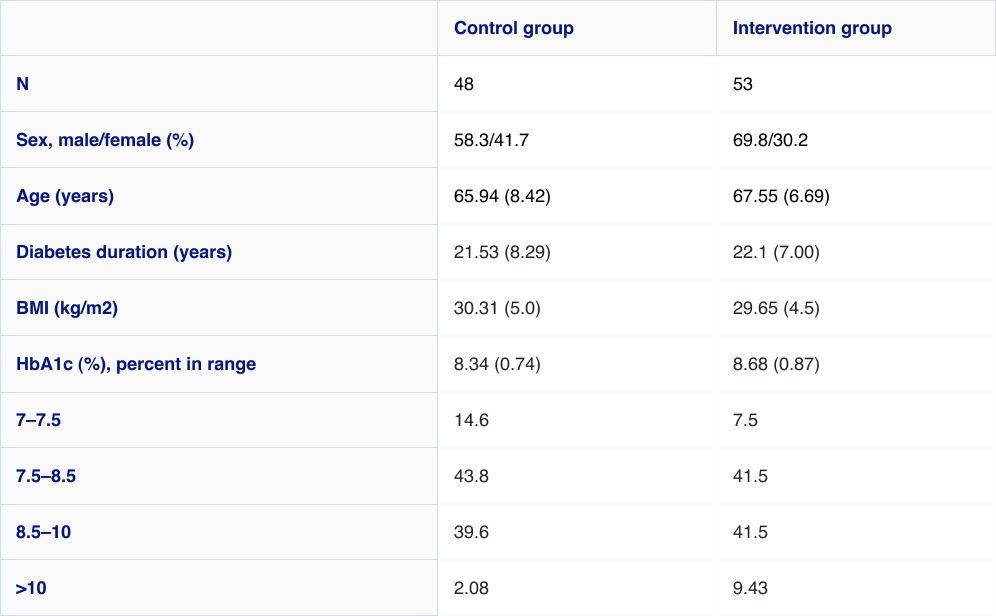
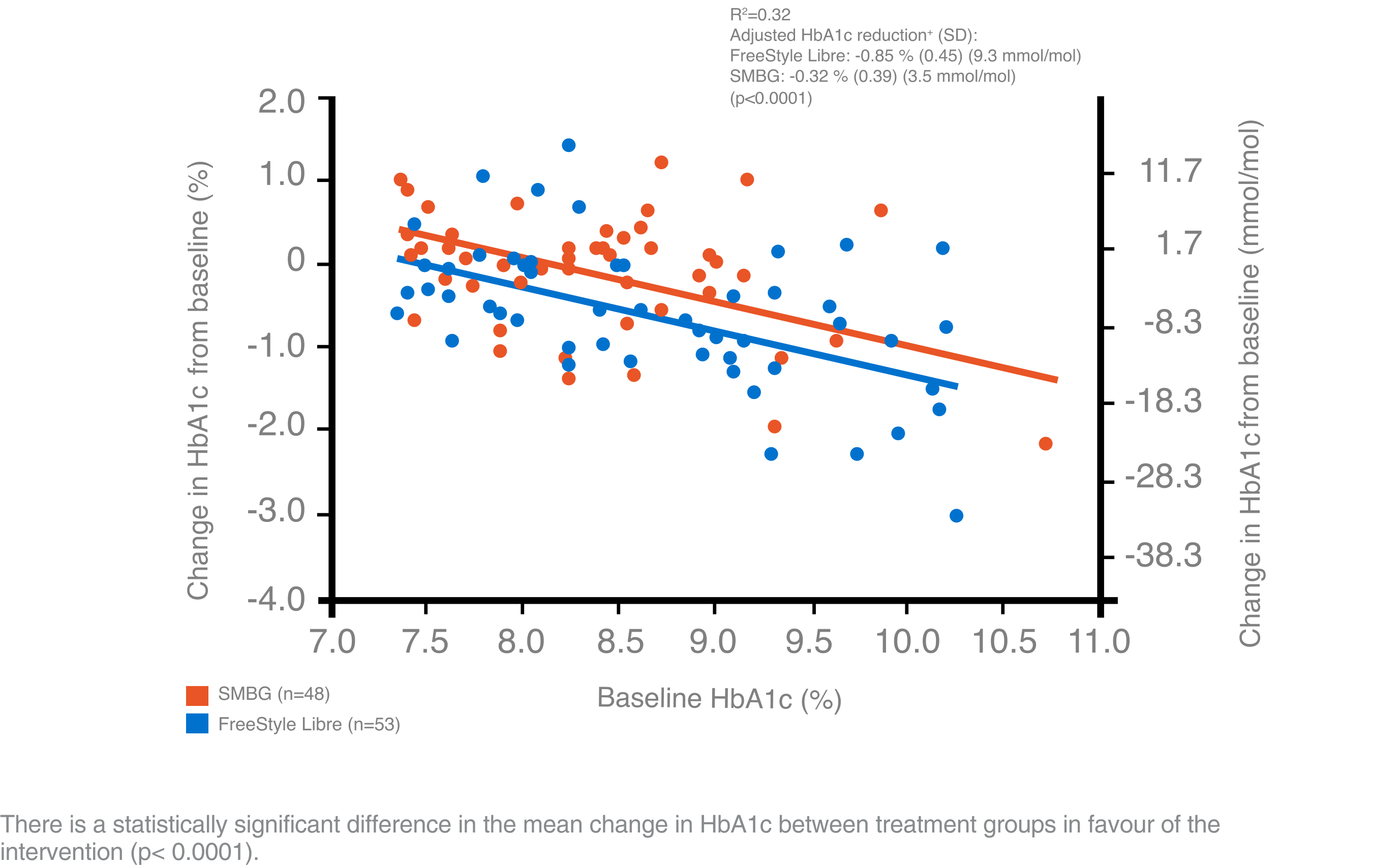
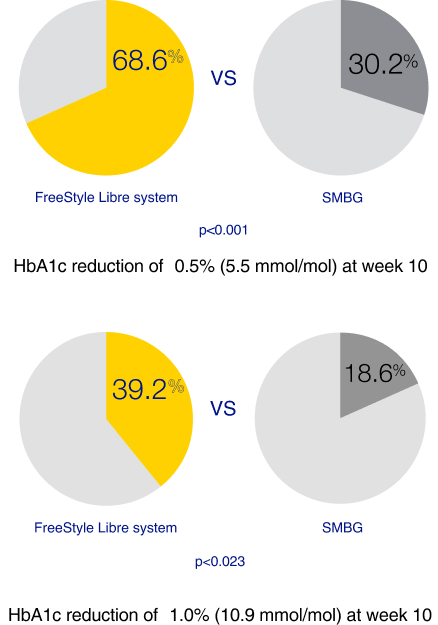
The open-label randomised (1:1) controlled trial demonstrates a high satisfaction and a significant reduction in A1c among users of the FreeStyle Libre system vs. self-monitoring of blood glucose (SMBG) without an increased frequency of hypoglycaemia.
The FreeStyle Libre system led to improved treatment satisfaction and better glycaemic control in patients with T2D on multiple daily injections (MDI).
.svg)