FreeStyle Libre Portfolio
The FreeStyle Libre Portfolio exists to empower those living with diabetes to make diabetes self-management choices and to help you, as their healthcare professional, to support them in their journey.
The FreeStyle Libre Systems
For patients looking for an easy way to confidently monitor their glucose levels2,3 | For patients looking for discreet4 continuous glucose monitoring (CGM) |
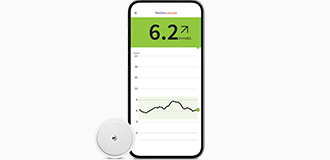 |  |
Manage diabetes with
Your patients using the FreeStyle Libre 2 system can see the impact of food, activity and medication on their smartphone◊ with a quick glance¤ to manage their glucose levels confidently.3 | Our most discreet4 & accurate
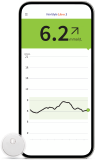
Your patients can know sooner, act faster and be ready to avoid hypos with our most reliable next-generation CGM6. Real-time glucose readings sent right to your patients’ smartphones.◊◊ |
Comparing the FreeStyle Libre sensors
| FreeStyle Libre 2 sensor7 | FreeStyle Libre 2 Plus sensor7 | FreeStyle Libre 3 sensor8 |
| Calibration needed | ||
| None | ||
| Wear duration | ||
| 14 days | 15 days | 14 days |
| Size | ||
| Small Only 35mm in diameter and 5mm in height | World's smallest and thinnest9 smaller than two stacked 5 Euro cents | |
| Sensor Reading Frequency | ||
| Every 1 min | ||
| Glucose data transfer | ||
|
|
|
| Optional glucose alarmsǁ | ||
| High, Low & Signal Loss | ||
| Sensor Data Storage | ||
| 8 hrs | 14 days | |
| Bluetooth range | ||
| 6 m | 10 m | |
| Paediatric indication | ||
| 4 years and older | 2 years and older | 4 years and older |
| Partnerships | ||
| NovoPen® 6 and NovoPen Echo® | None | mylife Loop Automated Insulin Delivery System |
References & Disclaimers
Images are for illustrative purposes only. Not real patient or Health Care Professional or data.
1. Data on file, Abbott Diabetes Care. Data based on the number of users worldwide for the FreeStyle Libre portfolio compared to the number of users for other leading personal use sensor-based glucose monitoring systems.
2. Haak, T. Diabetes Ther (2017): https://doi.org/10.1007/s13300-016-0223-6.
3. Fokkert M. BMJ Open Diab Res Care (2019): http://dx.doi.org/10.1136/bmjdrc-2019-000809.
4. Data on file, Abbott Diabetes Care, Inc.
5. Comparison based on publicly available information. Data on file. Abbott Diabetes Care.
6. CGM reliability defined by signal connection. Data on file, Abbott Diabetes Care, Inc.
7. FreeStyle Libre 2 User's Manual.
8. FreeStyle Libre 3 User’s Manual. FreeStyle Libre 3 demonstrates 7.8% overall MARD in accuracy to YSI.
9. Among patient-applied sensors.
◊ The FreeStyle LibreLink app is only compatible with certain mobile devices and operating systems. Please check the website for more information about device compatibility before using the app. Use of FreeStyle LibreLink may require registration with LibreView.
◊◊ The FreeStyle Libre 3 app is only compatible with certain mobile devices and operating systems. Please check our website for more information about device compatibility before using the app. Sharing of glucose data requires registration with LibreView.
¤ Glucose readings are automatically displayed in the FreeStyle LibreLink app only when your patients’ smartphone and sensor are connected and in range.
ǁ For FreeStyle Libre 2 system: Notifications will only be received when alarms are turned on and the sensor is within 6 meters unobstructed of the reading device. For the FreeStyle Libre 3 system: Notifications will only be received when alarms are turned on and the sensor is within 10 meters unobstructed of the reading device.
^ The sensor housing, FreeStyle, Libre, and related brand marks are marks of Abbott. mylife and YpsoPump are registered trademarks of Ypsomed AG. CamAPS is a registered trademark of CamDiab Ltd. Other trademarks are the property of their respective owners. For use of FreeStyle Libre 3 sensor with the mylife Loop, refer to the labeling provided with the mylife CamAPS FX app.
CamAPS FX is a CE marked medical device. CE 2797. Manufacturer: CamDiab Ltd; Level 4, Institute of Metabolic Science; Box 289, Addenbrooke’s Hospital, Hills Rd; Cambridge CB2 0QQ; United Kingdom. mylife™ YpsoPump® is a CE marked medical device. CE 0123. Manufacturer: Ypsomed AG, CH-3401 Burgdorf, Switzerland.
The sensor housing, FreeStyle Libre, and related brand marks are marks of Abbott. NovoPen® 6 and NovoPen Echo® Plus are registered trademarks owned by Novo Nordisk.
NovoPen® 6 and NovoPen Echo® Plus are CE-marked medical devices (0123). Manufacturer: Novo Nordisk.
ADC-94851 V1.0 09/24

