References & Disclaimers
FreeStyle, Libre, and related brand marks are marks of Abbott. Mylife and YpsoPump are registered trademarks of Ypsomed AG. CamAPS is a registered trademark of CamDiab Ltd. Omnipod and the Omnipod logo are registered trademarks of Insulet Corporation and used with permission. Other trademarks are the property of their respective owners.
1. NICE technology appraisal guidance TA943 (2023). Available at https://www.nice.org.uk/guidance/ta943. Accessed April 2024.
2. Crabtree TSJ, Griffin TP, Yap YW, Narendran P, Gallen G, Furlong N, Cranston I, Chakera A, Philbey C, Karamat MA, Saraf S, Kamaruddin S, Gurnell E, Chapman A, Hussain S, Elliott J, Leelarathna L, Ryder REJ, Hammond P, Lumb A, Choudhary P, Wilmot EG; ABCD Closed-Loop Audit Contributors. Hybrid Closed-Loop Therapy in Adults With Type 1 Diabetes and Above-Target HbA1c: A Real-world Observational Study. Diabetes Care. 2023 Oct 1;46(10):1831-1838. doi: 10.2337/dc23-0635. PMID: 37566697; PMCID: PMC10516256.
3. FreeStyle Libre 3 sensor is authorised to work with the mylife Loop automated insulin delivery system, including the mylife CamAPS FX app and mylife YpsoPump insulin pump. For use of FreeStyle Libre 3 sensor with the mylife Loop, refer to the labeling provided with the mylife CamAPS FX app.





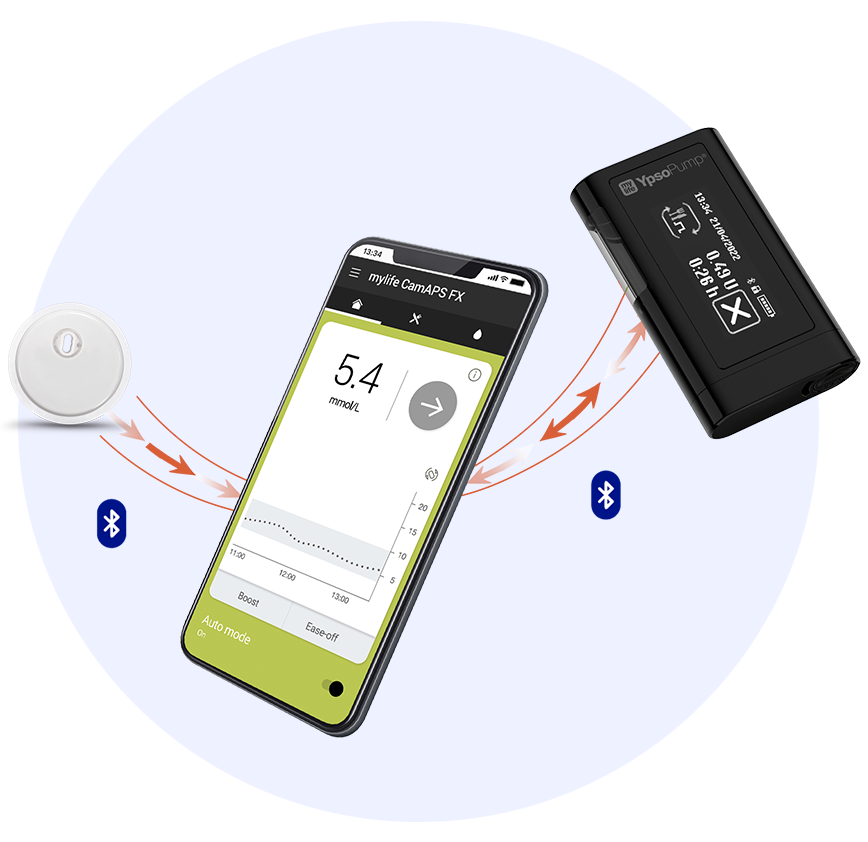
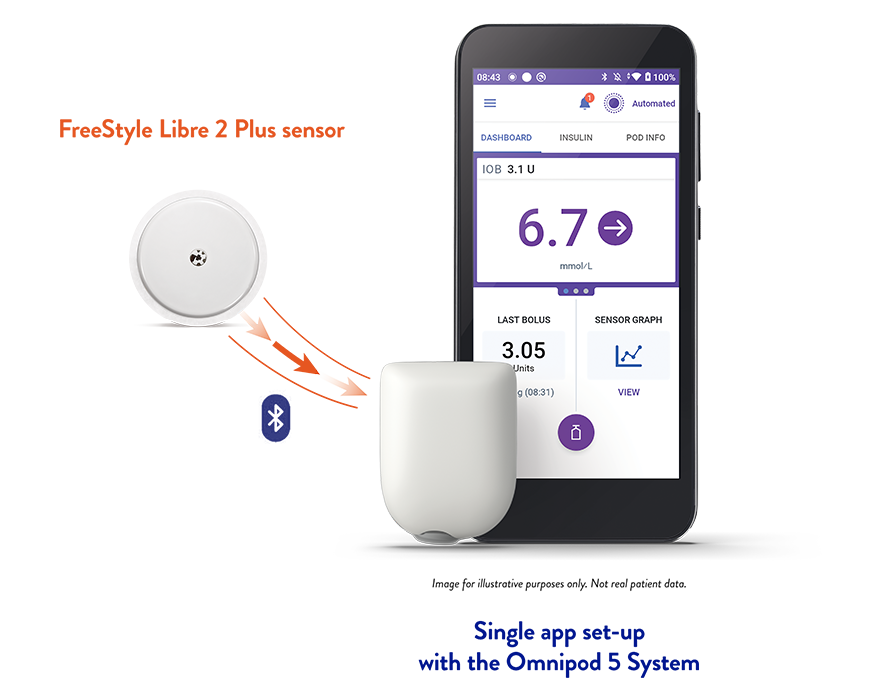
Stay connected